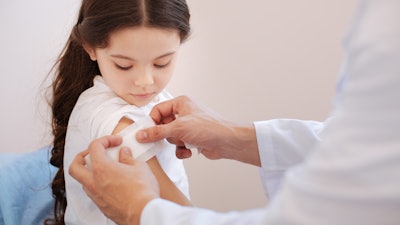
H.B. Fuller announces the launch of Swiftmelt 1515-I, its first bio-compatible product compliant in IMEA – India, Middle East, and Africa. The product is for medical tape applications to be used in stick-to-skin under unique climatic conditions, such as the high temperatures and humidity in the Indian sub-continent.
Swiftmelt 1515-I delivers effective high tack, secure and quick bonding, under high temperature and with shear resistance. The product, which shows excellent performance, balancing adhesion and ease of removal, was thoroughly tested and certified by ISO 10993-5 for cytotoxicity. Since it is not toxic to human cells, the adhesive can effectively be used in medical applications in hospitals and home care in stick-to-skin situations.
“This is a breakthrough from our H.B. Fuller’s R&D team in India, which has developed this new technology in our lab in Pune, in response to the IMEA market specifics to support advanced stick-to-skin product compliant for medical use – such as medical plasters or bandages,” says Manish Dwivedi, flexible materials, business manager for India. “Globally, our products have been used around the world in medical applications for more than two decades, enabling diverse new ideas and accelerating innovation for this important industry.”