apoQlar, a German medical technology company, today announced it has received FDA 510(k) Class II clearance for VSI HoloMedicine, a mixed reality software device enabling surgeons to plan complex procedures using the power of immersive 3D holographic technology. With this clearance, the USA becomes the 30th country for apoQlar to receive medical certification in. apoQlar will extend its distribution of VSI HoloMedicine in the USA for clinical use through its subsidiary in Miami, Florida, with availability expected in the second quarter of 2023.
Following this major achievement, apoQlar is now raising a Series A round, its first ever fundraising campaign, to scale VSI Holomedicine as the foundation of modern surgical care.
"With mixed reality, we are no longer bound to physical objects in a physical world. We can leverage digital objects and services on top of the real world for equal or greater utility and usually at a fraction of the cost. Mixed Reality is a completely new way for people, and in our case surgeons, physicians and technologists, to continue to experience the real world around them but with an entire virtual layer placed on top" says Sirko Pelzl, Co-Founder & CEO of apoQlar.
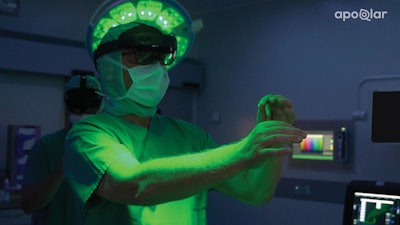
VSI HoloMedicine gives surgeons an almost "x-ray vision" perspective in surgical planning processes using 3D holographic technology. Physicians across any medical field can now plan surgeries in 3D and visualize medical data inside or outside of the operating room. Using Microsoft's HoloLens 2, a mixed reality head-mounted display (HMD), surgeons can transform otherwise flat CT, Angio CT, MRI, CBCT, PET, and SPECT sources into interactive 3D holograms. Sirko continues to say that "we are excited to finally offer VSI HoloMedicine® to improve surgical outcomes for patients and to provide surgeons with sophisticated surgical planning tools to enhance their overall planning process."
"FDA clearance marks a major milestone for us. We are a young company, but this serves as a true testament of our collective team mindset and diligence" says Liliana Duarte, COO of apoQlar. This is the latest achievement for apoQlar in their global expansion plan and serves as a tailwind for further market expansion efforts into South-East Asia, India, and the Gulf Coast States for 2023.






















